New 2016 AUA/SUO Clinical Practice Guideline Recommends Olympus NBI Technology for the Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer
Guideline Includes the World’s Only Patented Endoscopic Light Technology that Enables Effective Targeting of Biopsies Not Seen under White Light, Without the Use of Dyes or Drugs
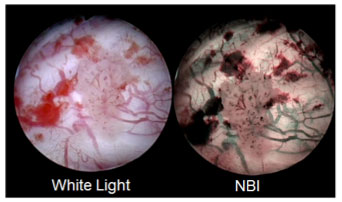
AUA/SUO Guideline includes NBI, the world’s only patented endoscopic light technology that, as compared to white light, visualizes lesions in additional 17 percent of patients, without the use of dyes or drugs.
CENTER VALLEY, Pa., (September 8, 2016) – Olympus, a global technology leader in designing and delivering innovative solutions for medical and surgical procedures, among other core businesses, announced today that the American Urological Association (AUA), together with the Society for Urologic Oncology (SUO), included the Olympus Narrow Band Imaging (NBI) technology in its newly released guidelines for diagnosing and treatment non-muscle invasive bladder cancer (NMIBC).
The AUA/SUO provides in the guideline recommendations on actions that should (or should not) be taken based on risk/benefit. The guidelines, approved by the AUA Board of Directors, are evidenced-based, meaning that a wide literature search for comparative effectiveness was conducted and reviewed.
“In a patient with NMIBC, a clinician may consider use of NBI to increase detection and decrease recurrence,” the guideline states, adding that enhanced cystoscopic techniques such as narrow-band imaging (NBI) “seem particularly valuable for diagnosis of urothelial carcinoma in the setting of positive cytology but negative white light cystoscopy.”
NBI is the world’s only patented endoscopic light technology that enables effective targeting of biopsies not seen under white light, without the use of dyes or drugs. NBI is not intended to replace histopathological sampling as a means of diagnosis. NBI enhances visibility of vascular structures on the mucosal surface. Unlike white light, which uses all colors in the spectrum, NBI uses only blue and green. Blue and green light are strongly absorbed by blood and appear darker than normal tissue. Blue light (415nm) highlights the shallow capillaries and green light (540nm) highlights deeper veins. NBI’s indication for the improved visualization of bladder cancer received FDA 510(k) clearance in 2015.
Based on a weighted average, the aggregated FDA-reviewed studies show NBI has visualized NMIBC lesions in:
- 17 percent additional patients when compared with white light
- 24 percent additional tumors
- 28 percent additional carcinoma in situ (CIS or difficult-to-detect flat lesions)
"NBI is a state-of-the-art technology for improved visualization of non-muscle-invasive bladder cancer," said Dr. Kenneth Kernen, MD, Beaumont Health System. “With its acceptance in the AUA guideline, it should quickly become the standard of care for the diagnosis, treatment and surveillance of bladder cancer."
“With just the touch of the button, NBI has made a big difference in how the most difficult-to-see lesions are visualized—without dyes that cause patient discomfort because of urine retention requirements, limitations on the number of uses in the indication, or additional equipment,” said Todd Usen, President, Olympus Medical Systems Group at Olympus Corporation of the Americas. “We are proud that the most important urological societies are recognizing the benefits NBI can bring toward improving quality of care, containing costs and improving patient satisfaction.”
Bladder cancer is the sixth most common cancer in the United States , with the highest lifetime treatment costs per patient of all cancers. White light cystoscopy misses small papillary tumors or CIS (difficult-to-detect flat lesions) at an estimated rate of 10-20 percent. Fifty-four percent of patients who go undiagnosed with CIS will progress to muscle invasive disease and require bladder removal (cystectomy) and a urinary diversion or neobladder. , Ninety-six percent of patients diagnosed early will survive five years later. Bladder cancer is known to recur 50 percent of the time.
The full guideline is available online at http://www.auanet.org/education/guidelines/non-muscle-invasive-bladder-cancer.cfm.
Olympus has created an informational site for better understanding of NBI technology, at www.simplyseemore.com, and also provides an app in the app store for viewing clinical pictures that outline the differences between white light and NBI for visualization of bladder tumors: https://itunes.apple.com/us/app/urology-nbi-atlas-by-olympus/id815796790?mt=8. For additional information or questions, please contact Olympus customer service at 1-800-848-9024.
# # #
About the American Urological Association
Founded in 1902 and headquartered near Baltimore, Maryland, the American Urological Association is a leading advocate for the specialty of urology, and has more than 21,000 members throughout the world. The AUA is a premier urologic association, providing invaluable support to the urologic community as it pursues its mission of fostering the highest standards of urologic care through education, research and the formulation of health policy.
About Olympus Medical Systems Group
Olympus Medical Systems Group, a division of global technology leader Olympus, develops solutions for healthcare professionals that help improve clinical outcomes, reduce overall costs and enhance quality of life for their patients. By enabling less invasive procedures, innovative diagnostic and therapeutic endoscopy, and early stage lung cancer evaluation and treatments, Olympus is transforming the future of healthcare. For more information visit Olympus at www.medical.olympusamerica.com.
i Li, K., Lin, T., Fan, X., Duan, Y., & Huang, J. (2013). Diagnosis of narrow-band imaging in non-muscle-invasive bladder cancer: A systematic review and meta-analysis. International Journal of Urology, 20, 602-609. http://www.ncbi.nlm.nih.gov/pubmed/23113702
ii SEER Cancer Statistics Factsheets: Bladder Cancer. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/statfacts/html/urinb.html
iii Sievert, K., Amend, B., Nagele, U., Schilling, D., Bedke, J., Horstmann, M., Stenzl, A. (Jun 2009). Economic aspects of bladder cancer: What are the benefits and costs? World Journal of Urology, 27(3), 295-300. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2694315/
iv Jichlinski, P., & Leisinger, H. (2005). Fluorescence Cystoscopy in the Management of Bladder Cancer: A Help for the Urologist! Urologia Internationalis, 74(2), 97-101. http://www.ncbi.nlm.nih.gov/pubmed/15756058
v Sylvester, R., Meijden, A., Witjes, J., Jakse, G., Nonomura, N., Cheng, C. Kurth, K. (2005). High-grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology, 66: 90–107 (Originally reference 29 of Li meta analysis). http://www.ncbi.nlm.nih.gov/pubmed/16399418
vi Li, K., Lin, T., Fan, X., Duan, Y., & Huang, J. (2013). Diagnosis of narrow-band imaging in non-muscle-invasive bladder cancer: A systematic review and meta-analysis. International Journal of Urology, 20, 602-609. http://www.ncbi.nlm.nih.gov/pubmed/23113702
vii SEER Cancer Statistics Factsheets: Bladder Cancer. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/statfacts/html/urinb.html
* Dr. Kernen is a paid consultant of Olympus.